Comprehensive tips for reliable and efficient analysis set-up
Comprehensive tips for reliable and efficient analysis set-up
Objective
This guide is designed to help you with your first set of projects on the Seven Bridges Platform. Each section has specific examples and instructions to demonstrate how to accomplish each step.
Also listed are common mistakes to avoid while setting up a project. If you need more information on a particular subject, our Knowledge Center has additional information on all of the Platform features. Additionally, our Support Team is available 24/7 to help.
Helpful Terms To Know
Tool – refers to a stand-alone bioinformatics tool or its Common Workflow Language (CWL) wrapper that is created or already available on the Platform.
Workflow / Pipeline (interchangeably used) – denotes a number of tools connected together in order to perform multiple analysis steps in one run.
App – stands for a CWL wrapper of a tool or a workflow that is created or already available on the Platform.
Task – represents an execution of a particular tool or workflow on the Platform. Depending on what is being executed (tool or workflow), a single task can consist of only one tool execution (tool case) or multiple executions (one or more per each tool in the workflow).
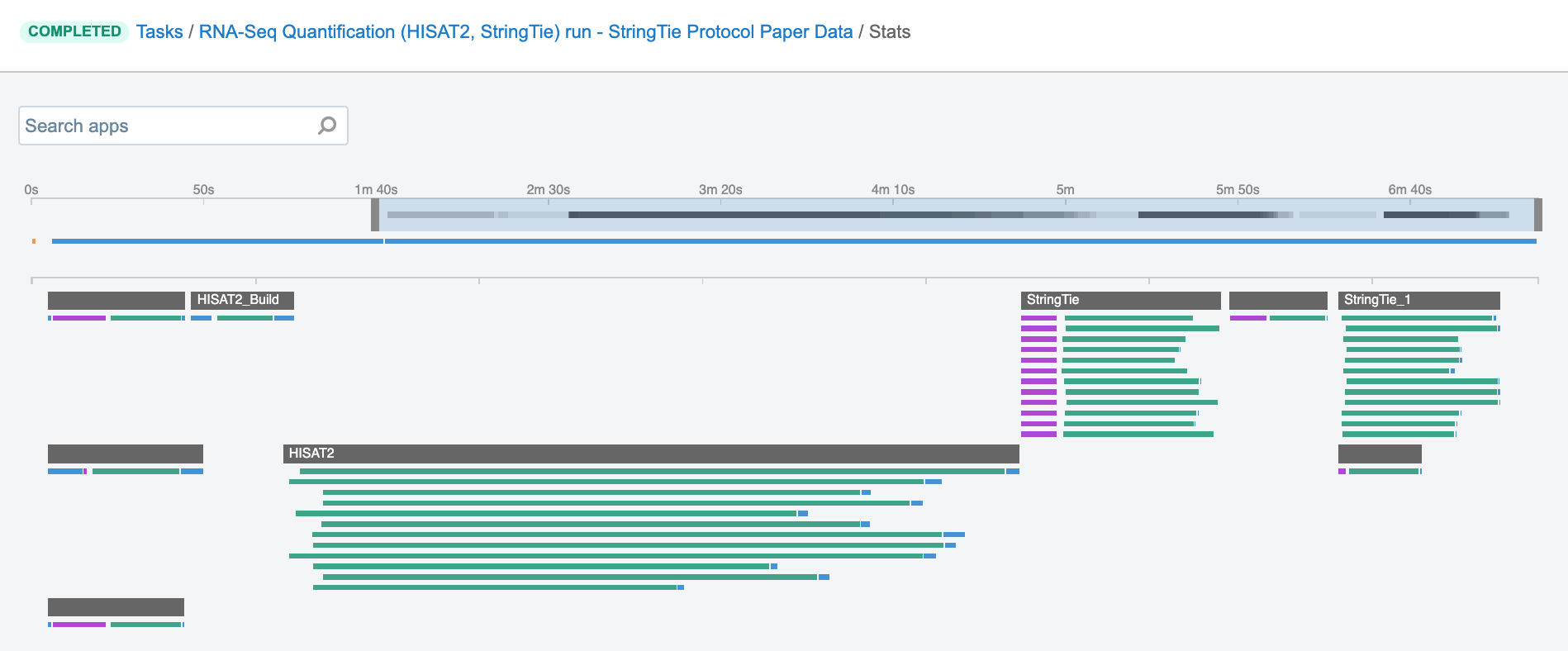
Job – this refers to the “execution” part from the “Task” definition (see above). It represents a single run of a single tool found within a workflow. If you are coming from a computer science background, you will notice that the definition is quite similar to a common understanding of the term “job” (wikipedia). Except that the “job” is a component of a bigger unit of work called a “task” and not the other way around, as in some other areas may be the case. To further illustrate what job means on the Platform, we can visually inspect jobs after the task has been executed using the View stats & logs panel (button in the upper right corner on the task page):
Figure 1. The jobs for an example run of RNA-Seq Quantification (HISAT2, StringTie) public workflow.
The green bars under the gray ones (tools) represent the jobs (Figure 1). As you can see, some tools (e.g. HISAT2_Build) consist of only one job, whereas others (e.g. HISAT2) contain multiple jobs that are executed simultaneously.
User Accounts & Billing
Depending on the collaboration needs, researchers can opt for creating a standard Single User account or an Enterprise account. If a relatively small number of researchers work on a specific project without need for additional levels of hierarchy and organization, a Single User account provides all of the necessary capabilities.
For research efforts split across multiple projects and across several teams which include a significant number of individuals, an Enterprise account is more suitable. In short, an Enterprise account is designed to mimic the organisational structure and provide means to efficiently manage collaborative groups and overall resource sharing.
Options for both Single User and Enterprise sign-up are available on-demand and can be established by getting in touch with the Seven Bridges team. If you are interested to learn more about subscription and billing options, you can contact our Sales Team and check out the overview of some basic, administrational steps. Since there are many different licensing and tier options, the most comprehensive and up-to-date information can be obtained through direct communication with the Sales Team.

Further Reading
Organization of work
Work performed on the Platform is divided into separate workspaces called projects (Figure 2). Each user can create projects and invite other registered users to be members of those projects. In addition, a variety of permission levels (e.g. admin, write, read-only) can be assigned to each project member.
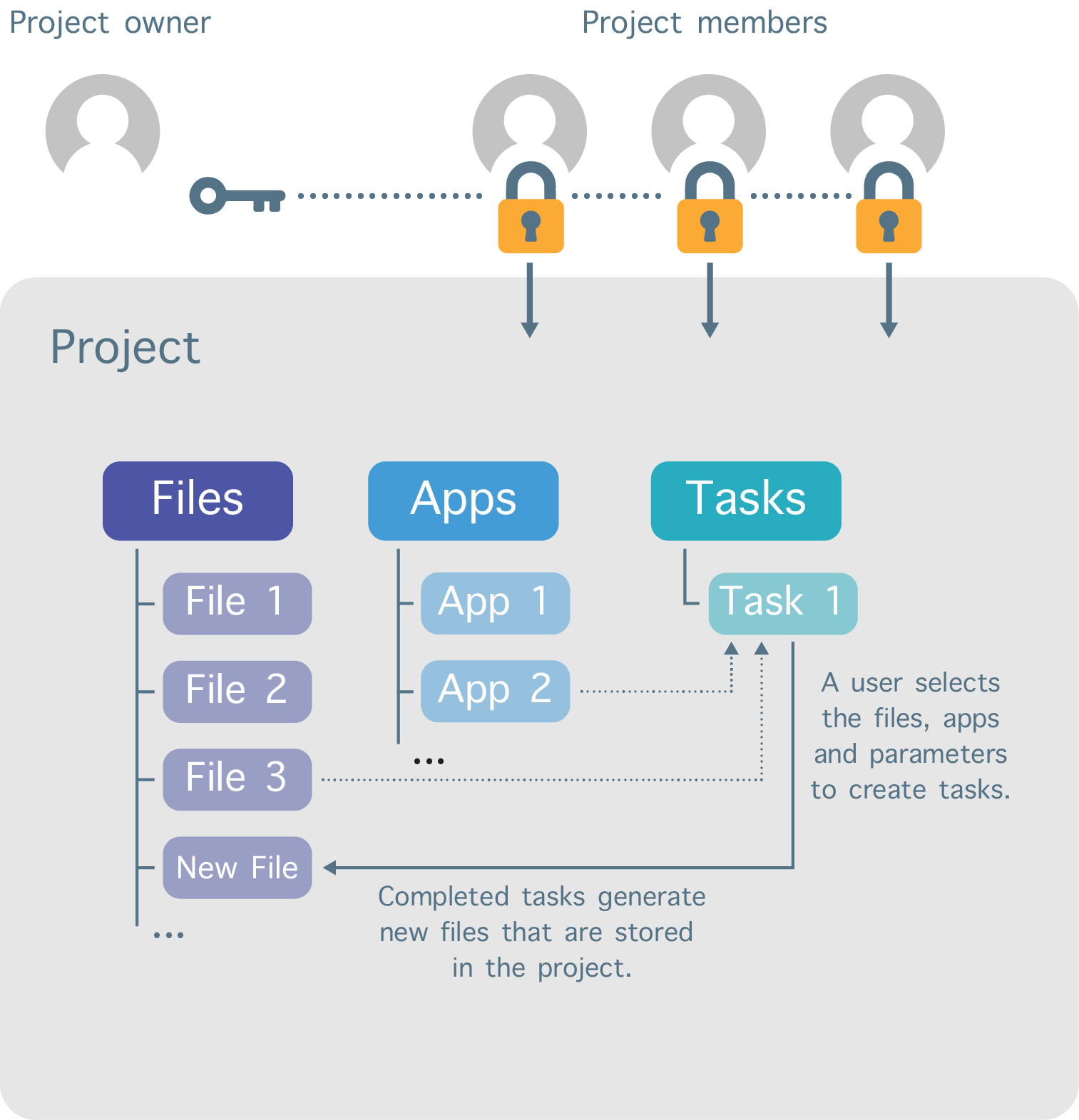
Figure 2. Project organization structure.
The user that creates the project sets the billing group to which the storage and compute costs are billed. The billing group for a project can be changed at any time.
However, some of these options may be constrained when using an Enterprise account. For example: If a user is a member of a Division (see here) in an Enterprise and they create a project, they will not be able to invite members from other Divisions to that project unless approved by their Division administrator. Similarly, the billing group may be automatically determined upon project creation for Enterprise accounts.

Further Reading
- Projects on the Seven Bridges Platform
- Add a collaborator to a project
- Change the billing group for a project
Tips for Running Tools/Workflows
In this section, you will find some of the most essential information on how to set up and run tools and workflows on the Platform. Creating CWL wrappers is not in the scope of this section (for that, see here); instead, we focus on Platform features which are fundamental to making your analysis efficient and scalable.
The content is structured such that it first offers information that is helpful for those researchers who want to use the public tools and workflows, and then it gradually advances to describe the features which can be used to adjust existing tools or develop new tools.
Start with the descriptions
For users who plan to execute any one of the hundreds of hosted tools or workflows from the Public Apps Gallery, the most important step is to thoroughly read the description. Even if you are familiar with the tools or you regularly run similar tools on your local machine, the description is where you will find the most useful, relevant, and up-to-date information on what you can expect in terms of the analysis design, expected analysis duration, expected cost, common issues, and more.
The Common Issues and Important Notes is an essential section in the description which gives insights into the known pitfalls when running the tool and should be studied thoroughly, especially when planning to run large scale analysis.
Although Seven Bridges aims to continuously improve the hosted public apps and seeks to educate researchers on their proper use, there are many places for error which can cause the tools/workflows to fail to successfully execute. Below is an example of how overlooking the description notes caused an unexpected outcome for a user:
Bioinformatics tools are usually designed to fail if certain inputs are configured incorrectly, and therefore terminate the misconfigured execution as soon as possible. However, in the case of VarDict variant caller, failing to provide the required input index files does not always result in the failure and can sometimes lead to infinite running.
This outcome occured when a user tried to process a number of samples by using the VarDict Single Sample Calling workflow. Previously, the user analyzed an entire cohort of samples with this workflow without any issues. Next, the user tried to apply the same strategy to another dataset in a different project. When setting up the workflow in the new project, the user copied the reference files to the new project and initiated the workflow with this new batch of files. However, the user left out the reference genome index file (FAI file) during the copying step, in turn producing several tasks which suffered from infinite running. Since the index files are automatically loaded from the project and not referenced explicitly as separate inputs, it was even more likely for this issue to go unnoticed.
The situation was additionally compounded by having a batch task (see Batch Analysis section for more details) which contained hundreds of children tasks. Because the user was not familiar with the related notes from the workflow description, they were not aware that the running tasks might in fact be hanging tasks. Had they known, they would have been able to terminate the run earlier, saving time and money.
In this case, the majority of the tasks failed early in the execution, producing minimal charges, but a few frozen tasks doubled the costs expected for the entire batch. We have since addressed the issues in this particular tool by introducing additional checks and input validations in the wrapper. Even though this is an extreme example, it demonstrates how descriptions can be critical to your tool working properly and can help you make informed decisions.
Test the workflow
For users planning a large scale analysis, it is recommended to begin by testing the workflow in a small number (1-5) of runs. By testing tools on a small scale, users can quickly make sure that everything works as expected, while minimizing costs. Additionally, testing the pipeline for the worst case scenario is one of the best ways to get more insight into the potential edge cases which could arise.
This can often be accomplished by running the analysis with the files of largest size in a given dataset. There are other situations where the highest computational load will correlate with the complexity of the sample content rather than correlating with size, which is more difficult to estimate.
Familiarity with the experimental design behind the sample files helps to create proper test cases for both size and complexity, which can keep potential errors to a minimum.
Specify computational resources
The term “computational instance” or “instance” refers to a virtual machine that can be chosen in order to adjust CPU and memory capacities for a particular application. The instances can be selected from the predefined list of Amazon EC2 or Google Cloud instance types.
For public apps, the instance resources have been pre-tested and defined by the Seven Bridges team. In most cases, the pre-defined instance types and default parameters will work for the majority of workflows, but users may need to optimize for certain input sizes or complexities, especially when scaling up workflows.
Small-scale testing can help inform if adjustments are needed in regard to the instance type(s) or tool parameters to be used throughout the analysis. There are two means by which instance selection is controlled:
- Resource parameters
- Instance selection
For the public apps, resource parameters are typically adjustable through the Memory per job and CPU per job parameters.
Setting the values for these will cause the scheduling component to allocate the most optimal instance type given these values. The following is an example from the task page:
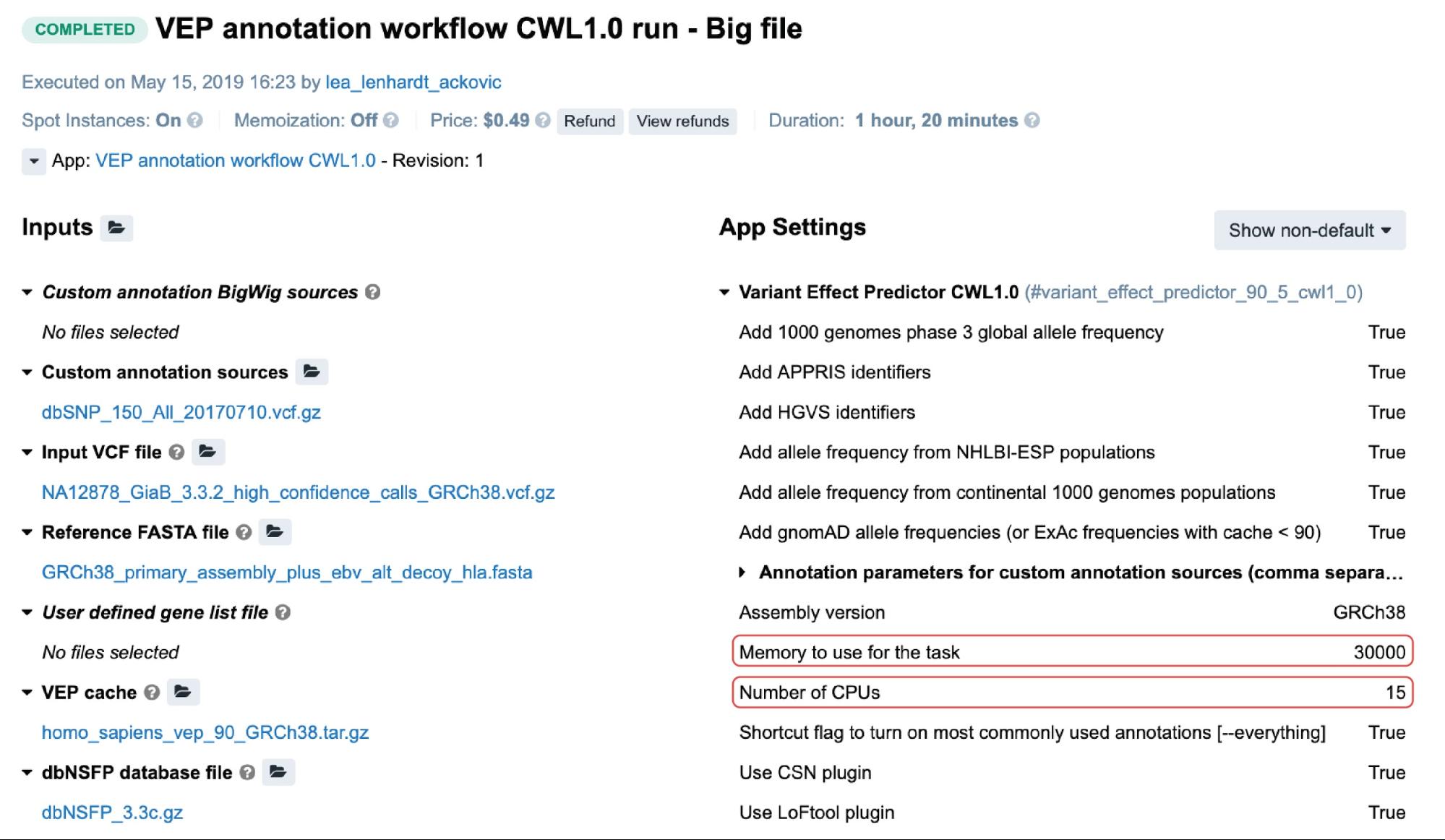
Figure 3. An example of a task containing the two resource parameters. The value for the memory is in megabytes.
In this particular case, c5.4xlarge instance (32.0 GiB, 16 vCPUs) was automatically allocated based on the two highlighted resource parameters (Figure 3). By checking the other instance types which may fit this requirement, it is shown that c4.4xlarge comes with 30.0 GiB and 16 vCPUs, which are the values closer to the given ones.
However, the Platform’s scheduling component also takes into account the price: because c5.4xlarge is more cost-effective than c4.4xlarge, it was given precedence.
Another way to control the computational resources is to explicitly choose an instance type from the Execution Settings panel on the task page:
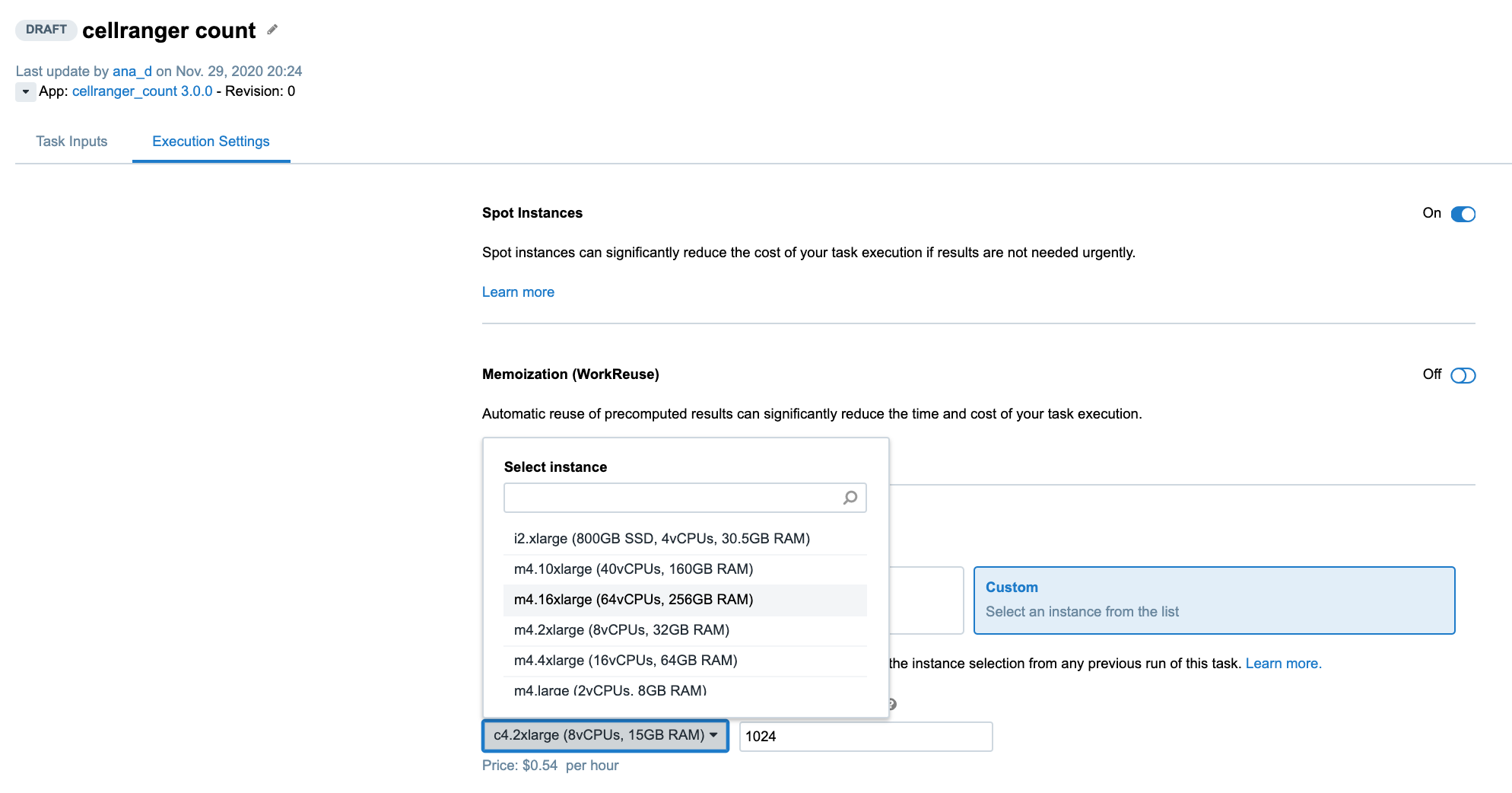
Figure 4. Selecting an instance type from the drop-down menu on the task page.
Learn about Instance Profiles
To be able to get the most out of available computational resources, it is essential to know how each individual tool behaves in different scenarios, such as when using different input parameters or different data file sizes.
One of the most efficient ways to identify patterns that perform well and use them to optimize future analyses is to monitor resource profiles.
The following is an example of the figures accessible from the Instance metrics panel found within stats & logs page (View stats & logs button is located in the upper right corner on the task page):
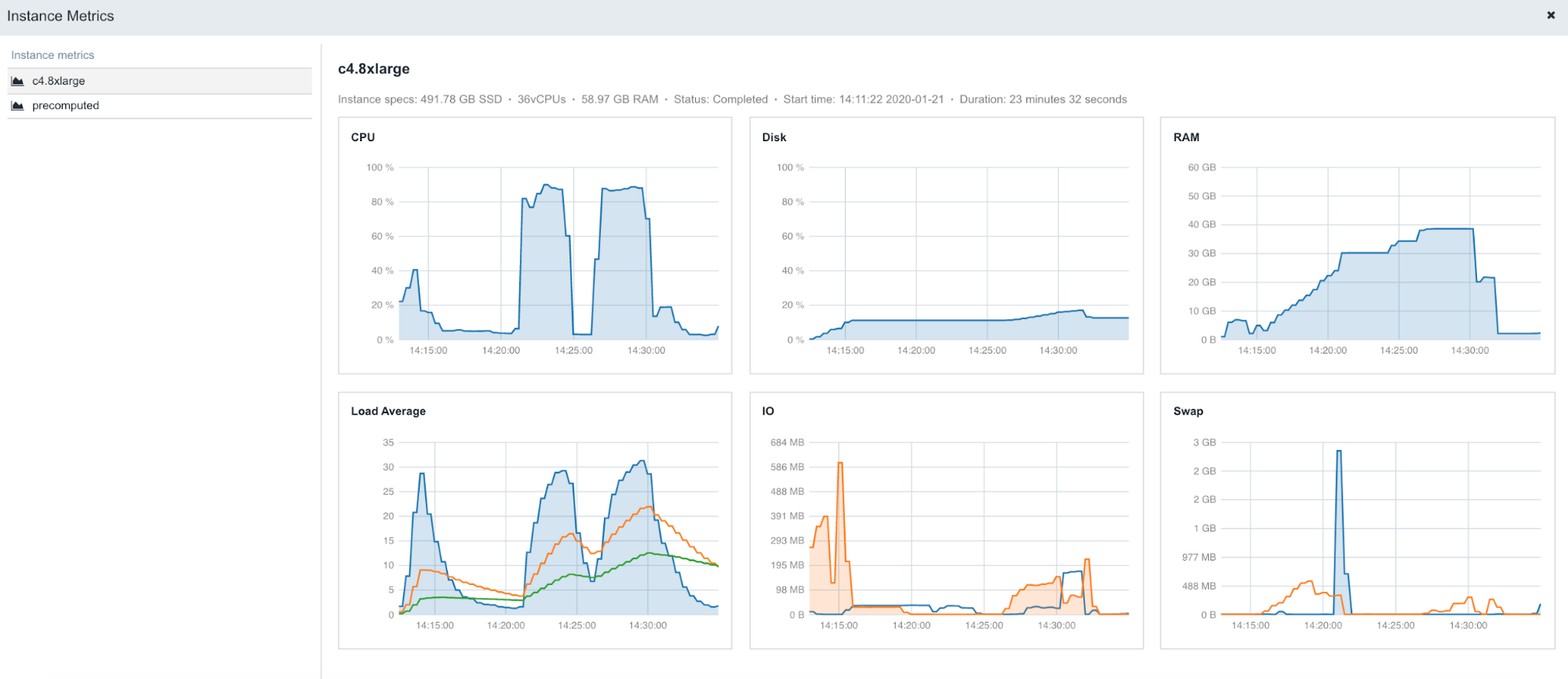
Figure 5. An example of instance profiles when running STAR 2.5.4b on Amazon c4.8xlarge (36 CPUs, 60GB of memory).
To learn more about Instance metrics, visit the related documentation page in our Knowledge Center.
Scale up with Batch Analysis
Batch analysis is an important step when analyzing larger datasets, typically consisting of many files for which it is desirable to apply the same processing methods throughout. We use batch analysis to create separate, analytically-identical tasks for each given item (either a file or a group of files depending on the batching method).
Two batching modes are available:
- Batch by File
- Batch by File metadata
In the Batch by File scenario, a task will be created for each file selected in the batched input. In the Batch by File Metadata scenario, a task will be created for each group of files that correspond to a specified value of the metadata (Figure 6).
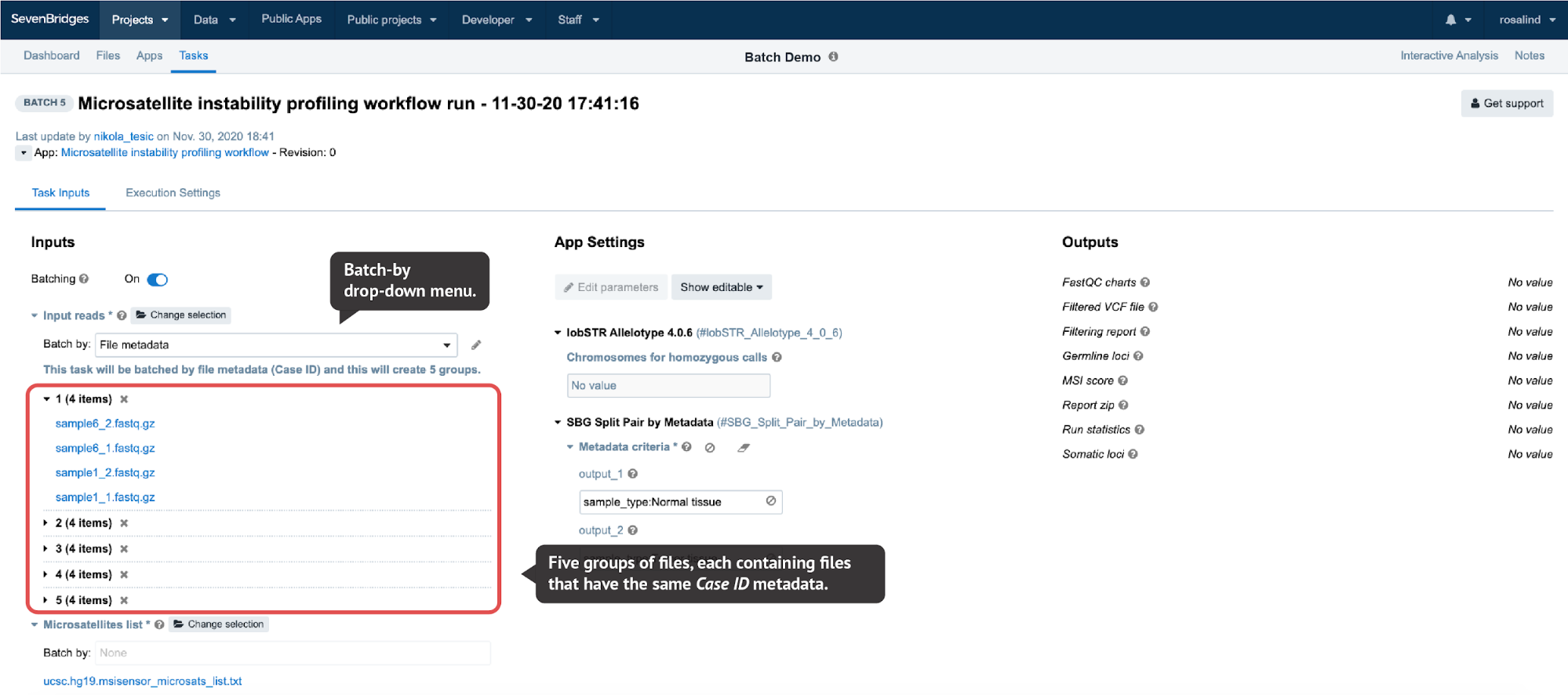
Figure 6. When File metadata is selected from the drop-down menu on the task page, a pop-up window is opened where users can select the metadata they want to batch by. Here, we provide raw reads for tumor-normal matched samples belonging to the five different cases as inputs to the MIcrosatellite Instability profiling workflow and by batching by Case ID metadata we automatically arrange the files into five groups. Each group contains two read pairs – one for the tumor and one for the normal sample of the same case. If we then run this batch task, five subtasks will be created, one for each group.
Batch analysis is robust in regard to errors or failures, meaning that failures in one task will not affect other tasks within the same batch of analysis. In other words, if we process multiple samples using batch analysis, each sample will have its own task. If one task fails, the other tasks will continue to process and can complete successfully, and the analysis will still be complete for most samples. Alternatively, it is possible to process multiple samples using another feature called Scatter (described in the following section)) and have all the samples processed within a single task. In contrast to batch analysis, a failure of any part of the scatter task (i.e. a job for an individual sample), will cause the entire task to fail and thus the analysis for all samples to also fail. In summary, batch analysis highly simplifies the process of creating a large number of the same tasks while allowing independent, simultaneous executions.
Important: Before starting large scale executions with Batch Analysis, please refer to the “Computational Limits” section below.
Parallelize with Scatter
Many common analyses will benefit from highly parallel executions, such as analyzing groups of files from specific disease types (e.g. TCGA BRCA).
The first factor that will determine the parallelization strategy is the user desires to execute the same tool multiple times within one computational instance, or instead to use multiple, independent instances to run the whole analysis in parallel for different input files (i.e. batch analysis). The feature which enables the former is called Scatter.
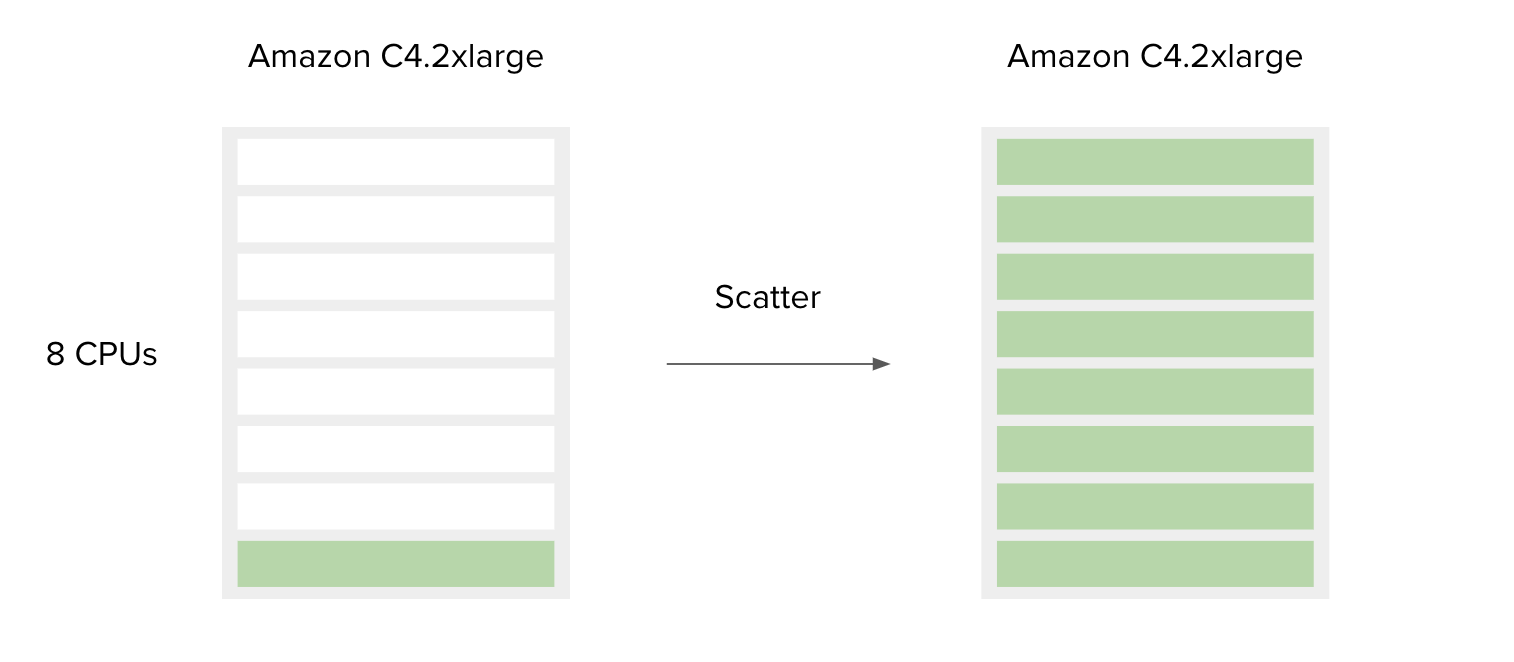
Figure 7. Simplified schematic explaining how scatter affects resource utilization. Green bars represent CPUs in use. If only one job that requires one CPU is run, the other CPUs will remain unused (left), whereas if8 such jobs are run, all CPUs will be utilized (right). The cost in both scenarios will be the same.
The following is a simple example to accompany Figure 7.
SBG Decompressor is a simple app used for extracting files from compressed bundles. It requires only one CPU and 1000 MB of memory to run. By default, the Amazon c4.2xlarge instance is chosen by the Platform since the requirements for CPU and memory of this tool are less than or equal to the c4.2xlarge capacities.
This means that if a user has only one file to decompress, most of the resources on the c4.2xlarge instance will remain unused. However, if a user has multiple files to process, the best way to do this is to use the Scatter feature. By doing so, the additional instance resources are also utilized. By design, scattering will create a separate job (green bar) for each file (or a set of files, depending on the input type) provided on the scattered input.
Another common application of the scatter feature is seen when using the HaplotypeCaller tool to call SNPs and indels across large genomic intervals (e.g. entire chromosome set). Instead of searching through the whole genome, the HaplotypeCaller tool has an option which allows for defining specific genomics regions (e.g. intervals) within which variants will be called.
With this in mind, it is possible to set up the processing such that each chromosome is run independently from each other. This is a great example of a task that will benefit from scattering, as users can run the tool in parallel across all chromosomes.
By choosing the “include_intervals” input from the Scatter box (Figure 8), the tool will perform scattering by the genomic intervals input, which will result in an independent job for each provided interval file (usually BED or TXT format which contains interval name along with the corresponding start and end genomic coordinates).
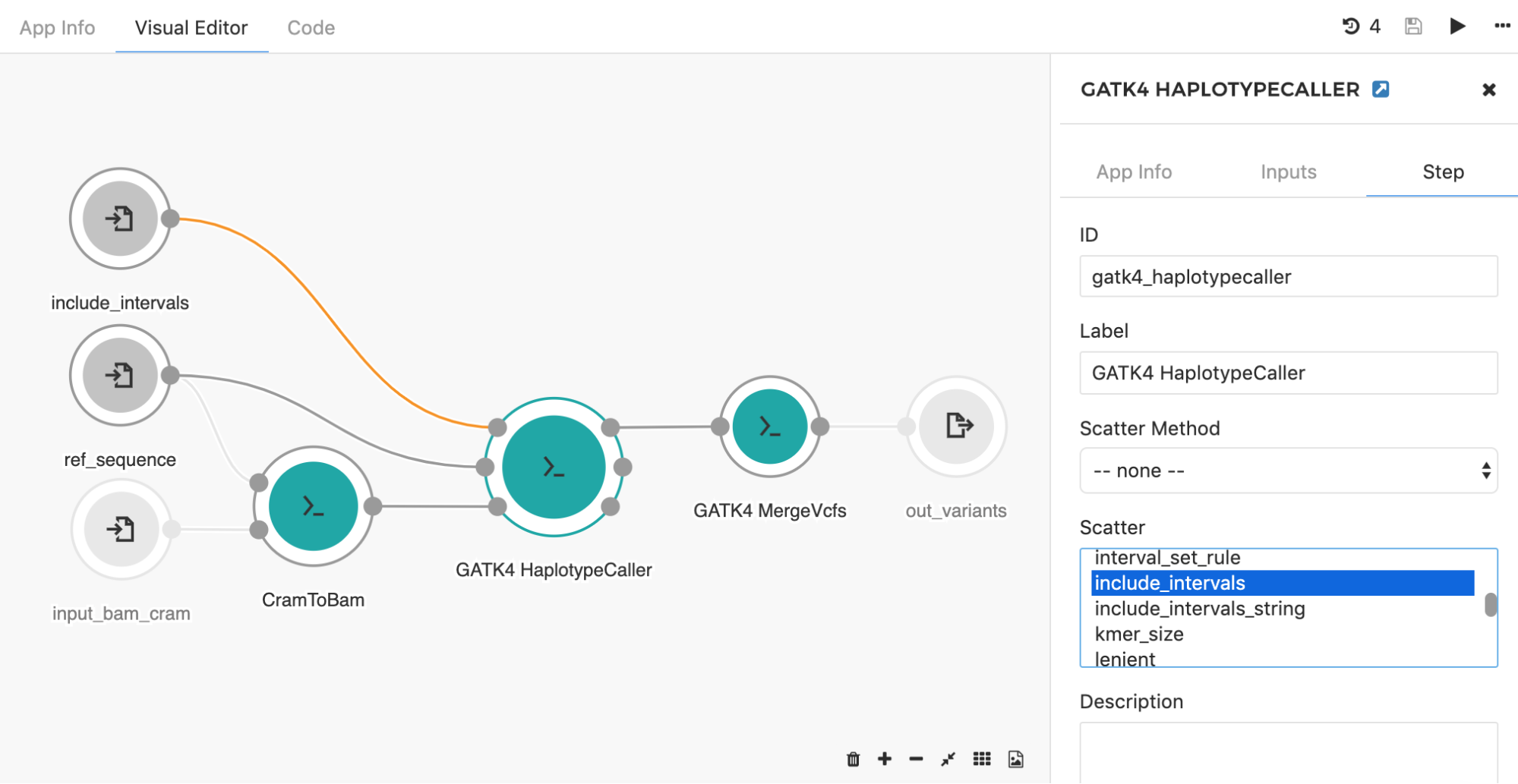
Figure 8. To set scattered input, double click on the tool of interest and select Step in the right-hand panel. The input you want to scatter can be selected under the “Scatter” section. Additionally, if that particular input is connected directly to the input node (there are no preceding tools in between), the input node needs to be modified (also by double clicking on the node) to accept an array of objects originally defined (e.g. array of files instead of one file). Once the input node is adjusted, the connection will turn orange. Here, GATK4 HaplotypeCaller input include_intervals is scattered, meaning that the independent job will be executed for each file provided on this input.
There are situations where it is desirable to create one job per multiple input files. To accomplish this, a way to bin the input files into a series of grouped items is needed.
A good example of this is when an input sample has paired-end reads, and users want to create jobs for each sample, not each read. In this case, SBG Pair FASTQs by Metadata tool (available as a public app) can be used to create the group for each pair, and this tool should be inserted upstream of the tool on which you will apply scatter in your workflow.
Recalling the comparison between Batch Analysis and Scatter from the previous section, it may appear that these features are alternatives to one another. Even though they are intended for somewhat similar purposes, Scatter and Batch Analysis are mostly used as complementary features – they can be applied to the same analysis, such as in the HaplotypeCaller example above.
The workflow already contains the HaplotypeCaller tool which is scattered across intervals. If we run the workflow and batch by input_bam_cram input, we create an individual task for each BAM/CRAM input file.
It is worth mentioning that many workflows available from the Public Apps Gallery already contain scattered tools. Hence, if a batch task is executed with those workflows, it efficiently accomplishes parallelization on both levels – within the task (with Scatter) and across multiple instances (with Batch Analysis).
One example of this is the Bismark Analysis workflow that takes raw reads (WGBS or RRBS) as inputs, performs sequence alignment, and outputs methylated sites. Prior to the alignment steps, low quality reads along with adapters are filtered out using the TrimGalore! tool that is not resource-intensive, but requires more time to do the work.
To compensate for that, the reads were split into chunks and TrimGalore! was run in parallel for each chunk using Scatter. Doing so managed to significantly drop the time spent in this step, while utilizing all the available resources within the selected instance type.
Unlike in the previous example where already-existing intervals were leveraged in order to enable parallelization, the Bismark Analysis pipeline goes one step further – pinpointing the app with a poor resource usage and inventing the logic that enables the application of Scatter. Finally, running this workflow in a batch mode will even further optimize the time spent for the analysis.
More details about all this topic can be found in the related blog post – Optimized Workflow for Bisulfite Sequencing Data Analysis.
Configuring default computational resources
In a previous section, this guide reviewed methods for controlling the computational resources from the task page, where you can choose an instance for the overall analysis on-the-fly.
While in many cases this is the recommended method, customizing the workflow for a specific scenario or optimizing it with Scatter will sometimes require setting up default instance types.
This can be configured from the tool/workflow editor by using the instance hint feature. This feature allows users to 1) select the appropriate resources for the individual steps (i.e. tools) in a workflow, and 2) define the default instance for the entire analysis (either a single tool or a workflow).
The user who creates the analysis can choose to set the default instance if they are confident that their analysis will work well on a particular instance and they do not want to rely on the user settings or the Platform scheduler.
However, if the default instance is not set, the user can select the instance for the analysis from the task page (as described above), or the user can leave it to the Platform scheduler to pick the right instance based on the resource requirements for the individual tool(s).
To set up an instance hint (i.e. chose a specific instance type) on the particular tool in the workflow, this can be done by entering the editing mode, then double-clicking on the node and selecting Set Hints button under the Step tab as follows:
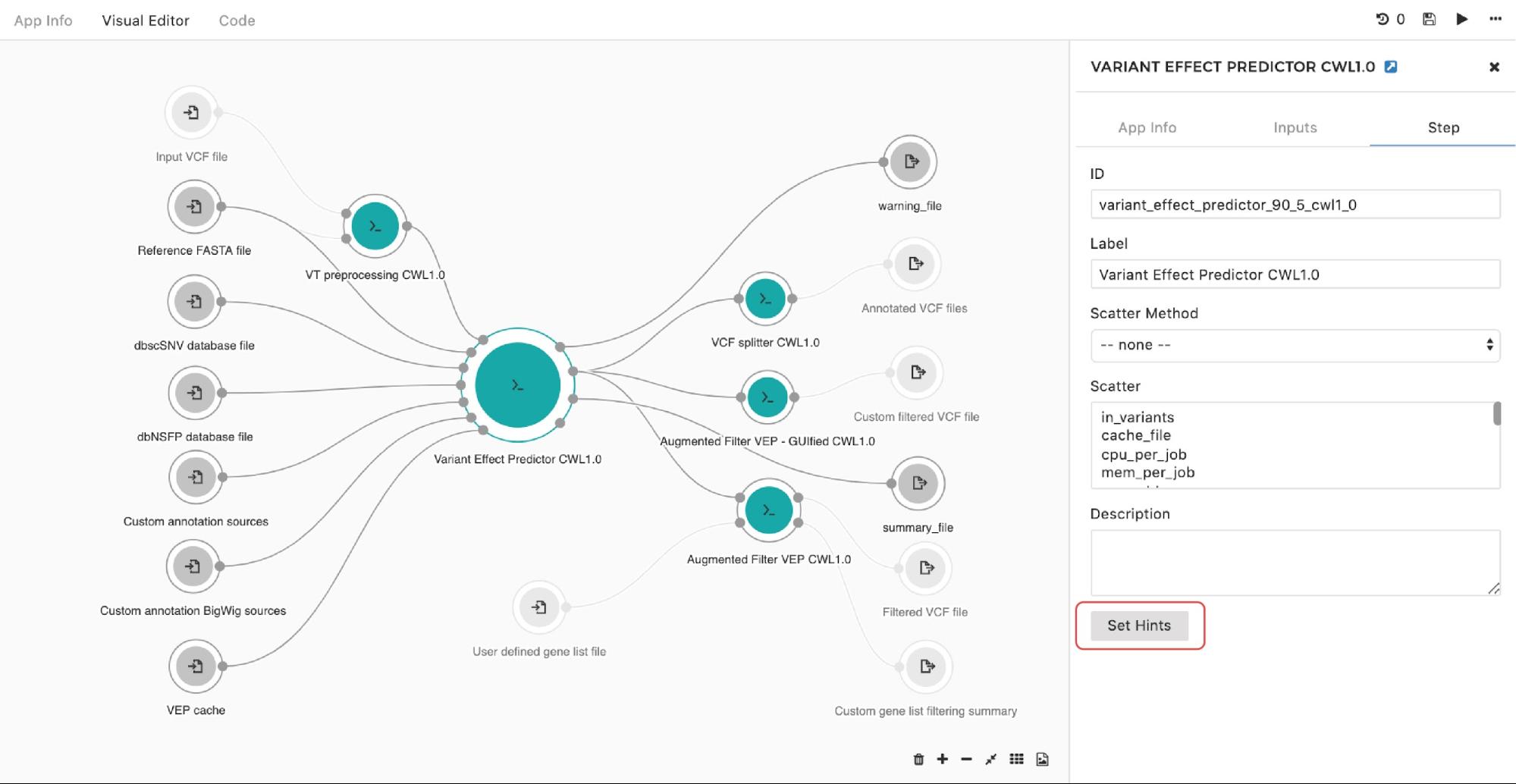
Figure 9. Setting instance hints for a particular tool in the workflow.
A configuration pop-up window will appear, then choose users can select instance types. This also applies for managing the instance hints for the whole workflow.
The only difference is that we get to the pop-up window through the drop-down menu in the upper right corner in the editor as shown in Figure 10:
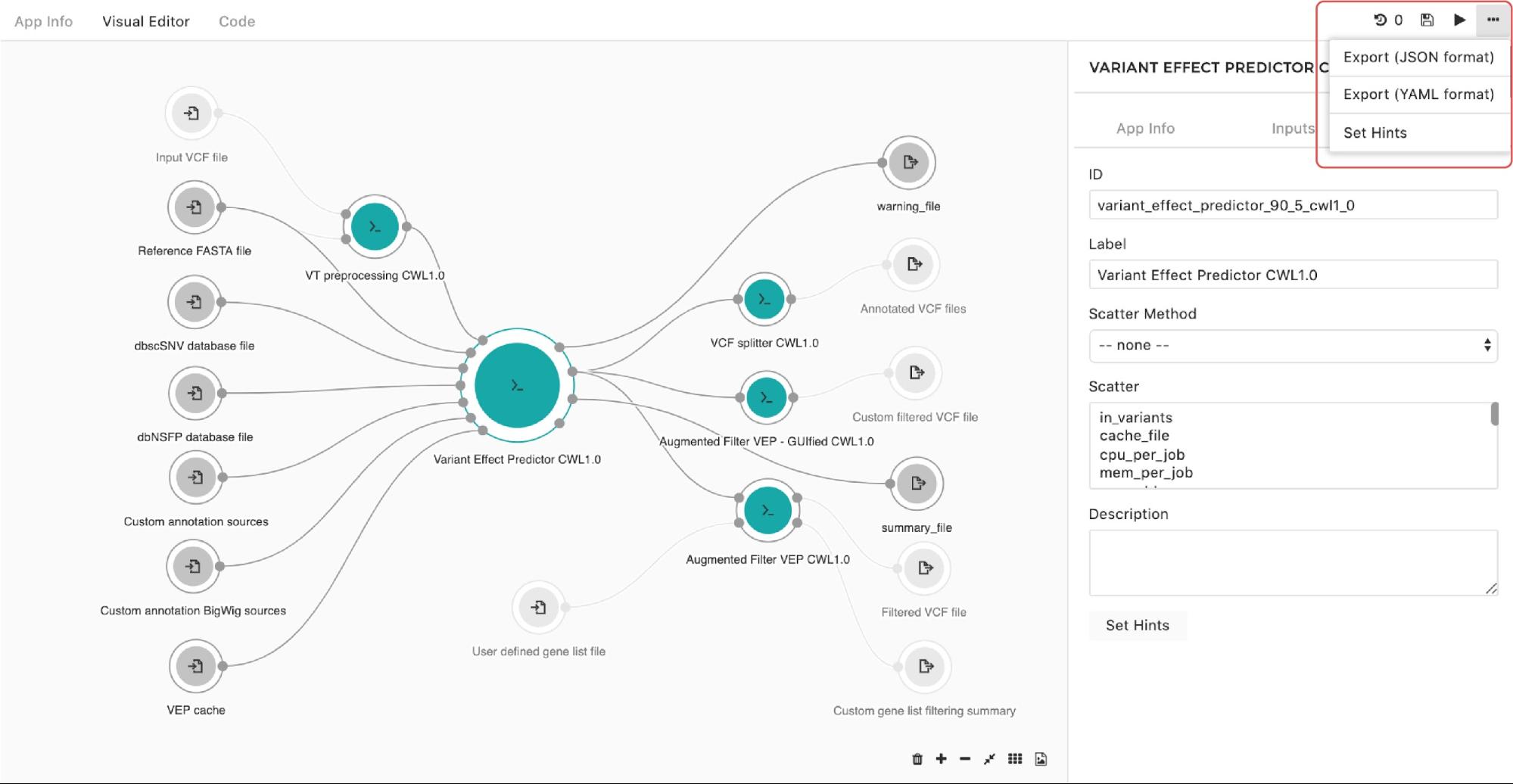
Figure 10. Setting instance hints for the whole workflow.
Finally, to set the instance hints for an individual tool, users can edit the tool and scroll down to the HINTS section shown in Figure 11:
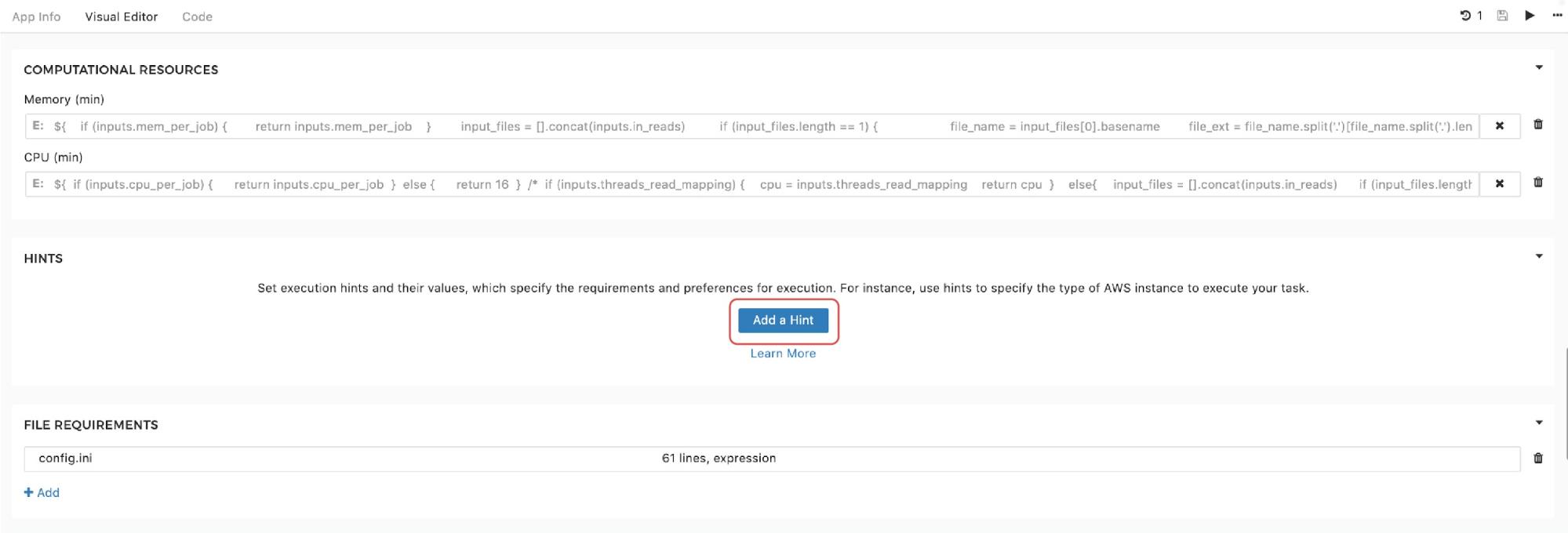
Figure 11. Setting instance hints within tools.
Recalling the resource parameters description, you may wonder what happens if the resource parameters are set and an instance hint is configured within the same tool. In this case, the scheduler will prioritize the instance hint value and will allocate the instance based on that information. However, the information about resource parameters needed for one job is not completely ignored.
Namely, if a workflow is about to be run, and the set-up is such that multiple jobs can be executed in parallel on the same instance (i.e. we use Scatter feature), resource parameters are also taken into consideration.
These parameters are not used for the instance allocation, but they determine the number of different jobs that can be “packed” together for simultaneous runs on the given instance. A detailed explanation of the scatter feature that enables this is provided in a previous section.
Further analysis and interpretation of your results
On the Seven Bridges Platform, users can further analyze their data by leveraging Python notebooks or R interactive capabilities by using the Data Studio feature.
With the Data Studio, users can easily access the files in their project for use in an R- or Python-based environment, without the need to download them to your local machine, and with the added flexibility of choosing computational resources.
Getting started
To access Data Studio:
- Navigate to the Data Studio tab.
- Once on the landing page, click the Create your first analysis button which will open an analysis set-up dialogue box (Figure 13)
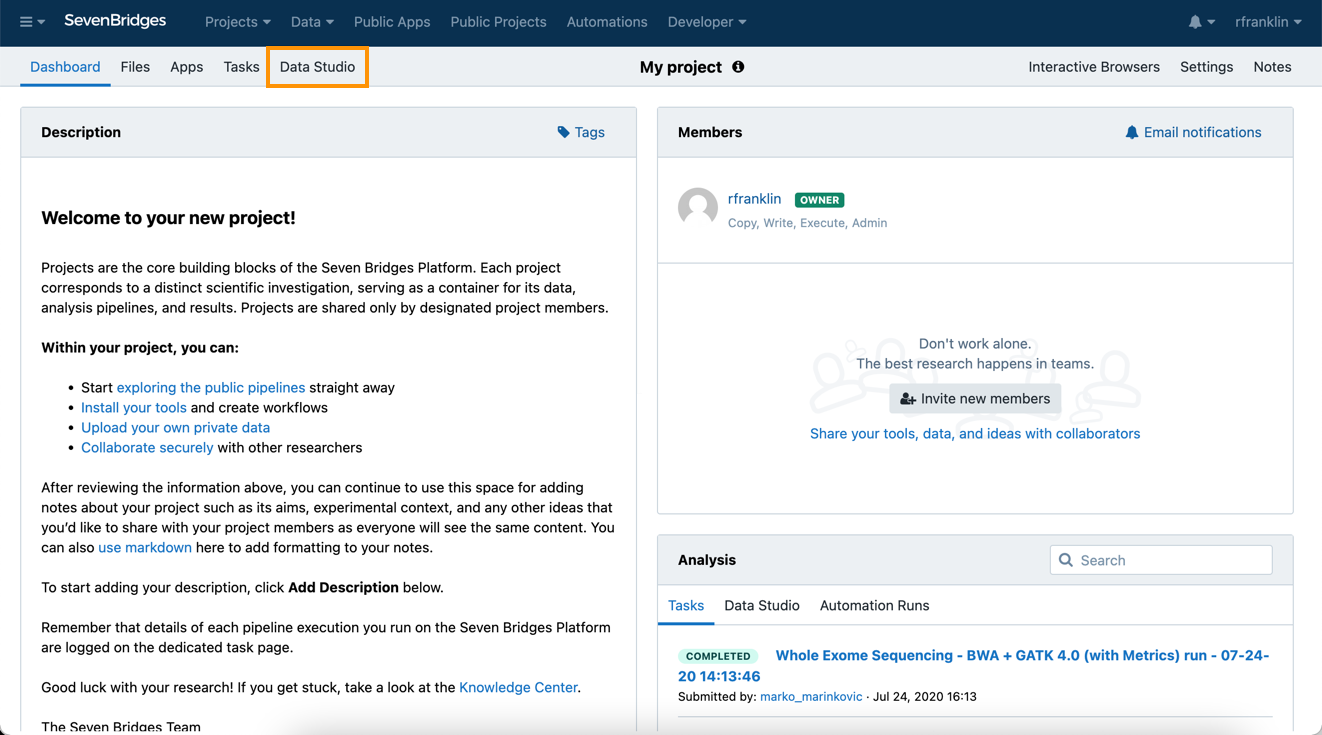
Figure 12. Navigating to the Data Studio.
There are two computing environments available (Figure 13):
- JupyterLab
- RStudio
There are several Amazon Web Services (AWS) or Google instance types that users can choose from. If an AWS location was selected at the time of project creation, then users will see AWS instance options, likewise for when Google is set as the project location.
It is shown in Figure 13 that there is a drop-down menu where users can configure the environment: meaning that users can choose one of the Docker images with pre-installed Python and R packages. The default image contains the most common packages needed for scientific analysis and visualizations.
In addition, Suspend time can be configured as a safety mechanism to enable automatic termination of the instance after a period with no activity.
This feature can be turned off completely if a user plans a longer interaction and does not want to risk termination. We recommend that users carefully check the Location and Suspend Time settings before starting the analysis, especially if running an existing one which already has its own configuration (Figure 14).
After setting up the preferences, click on the Start button to spin up the instance. It may take several minutes for the instance to initialize.
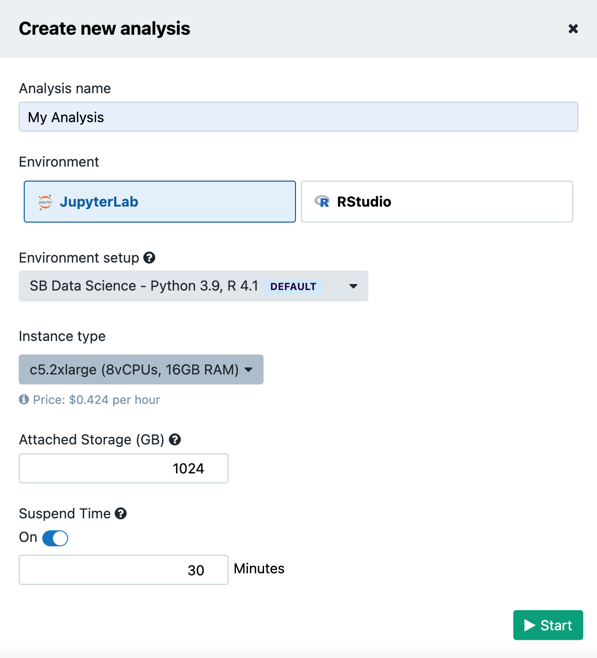
Figure 13. Setting up the interactive analysis in Data Studio.
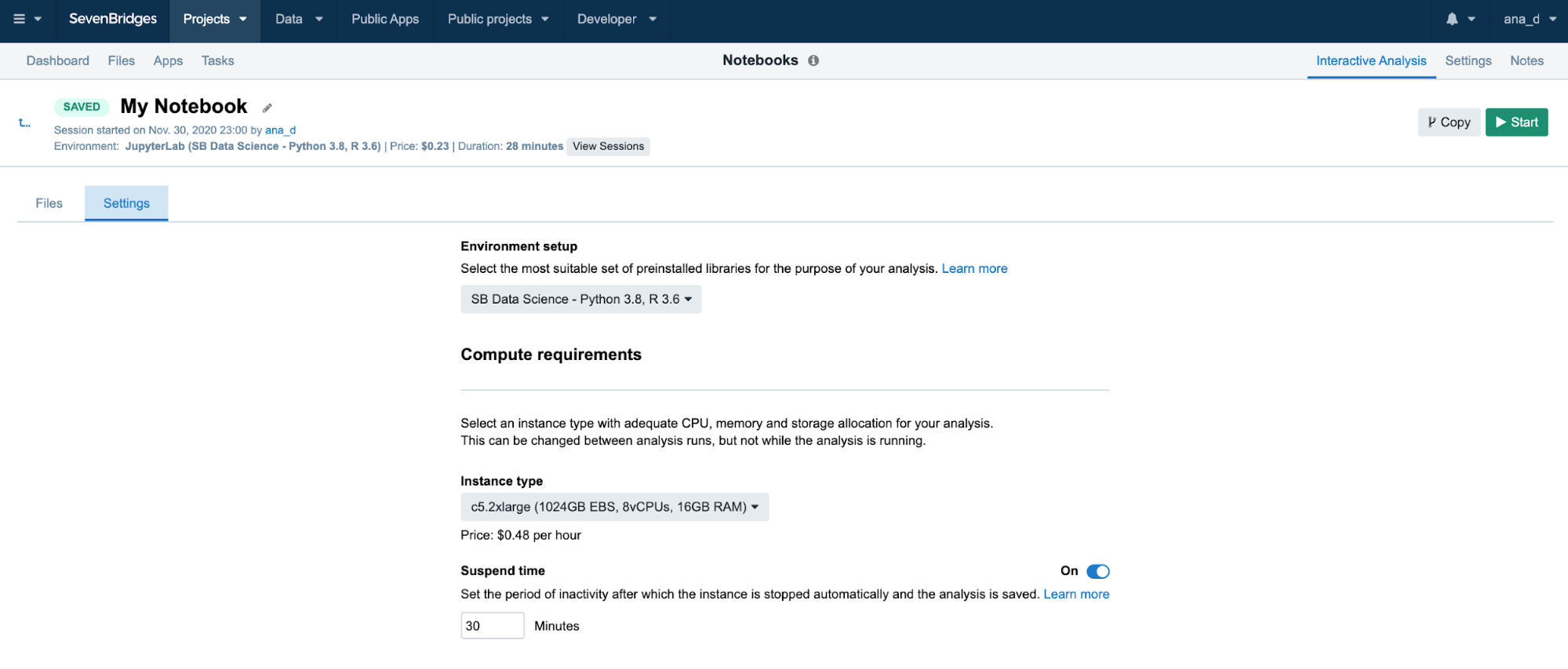
Figure 14. Analysis settings panel. The computational requirements can be edited when the analysis is inactive.
JupyterLab environment
Once the instance is ready, the editor will open. From there, users can move forward with their analysis.
Accessing the files
The files which users can analyze within the notebook are:
- files present in the analysis – either files uploaded directly in the analysis workspace (the home folder for the interactive analysis) or files produced by the interactive analysis itself, and
- files present in the project
The list of files available in the analysis is displayed in the left-hand panel under the Files tab. This is a list of items in the /sbgenomics/workspace directory, which is the default directory for any work that users perform during a session.
To control the content in this directory (create new folder, upload files etc.), users can utilize the workspace toolbar located above this panel.
However, if you are interested in using an interactive analysis to access the data from your project, List all the files in the /sbgenomics/project-files directory and select the one of interest (see cell [4] in Figure 16).
This path is immutable across different projects. Therefore, if a user copies the analysis to another project and they have referenced their file this way, it will function the same way in the new project.
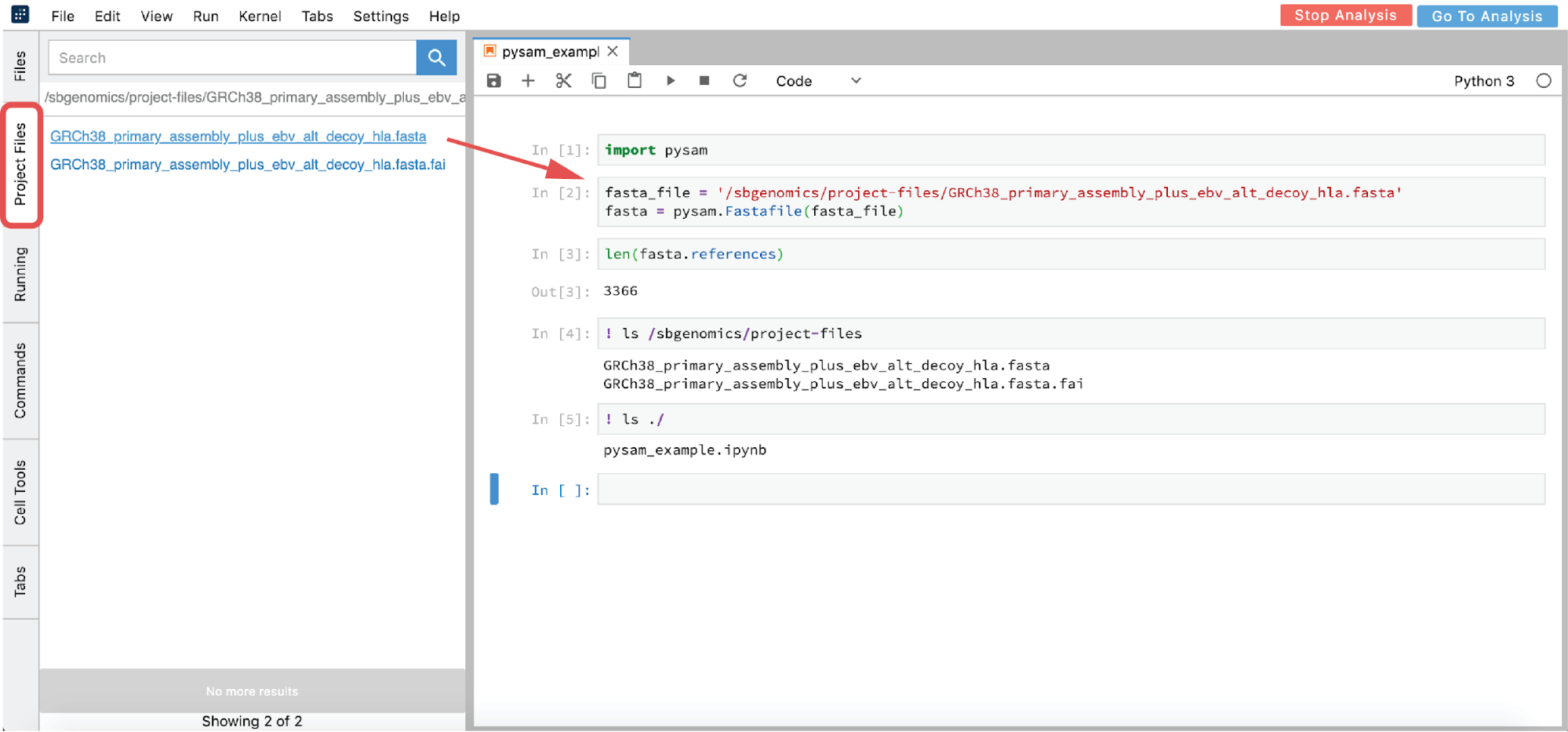
Figure 16. Python 3 Notebook example.
In the previous example, “!” was used in two notebook cells to switch from Python to shell interpreter, and hence denote that these cells should be executed as shell commands.
If there is a need to use shell more intensively (such as for installation purposes, etc) the notebook environment can become impractical. Fortunately, there is an option to mitigate this: a terminal can be opened from the launcher page.
Saving the created files
Finally, when the user has completed the analysis and wants to save the results to their project, the next step is to copy the files to the /sbgenomics/output-files directory from the terminal.
Please note that only smaller files (e.g. .ipynb files) will continue to live in the analysis after it has stopped. Hence, all other needed files need to be saved before you decide to terminate the session.
RStudio environment
For users who prefer to conduct their analysis using RStudio, they should follow the identical procedure from above with an exception of instead selecting RStudio in the analysis creation dialogue box.
Accessing and saving the files in RStudio
Unlike the JupyterLab environment, RStudio environment does not offer convenient file handling from the GUI. However, the same paths that we mentioned in previous sections are also used in RStudio:
- Project files directory:
/sbgenomics/project-files - Workspace directory:
/sbgenomics/workspace - Output directory:
/sbgenomics/output-files
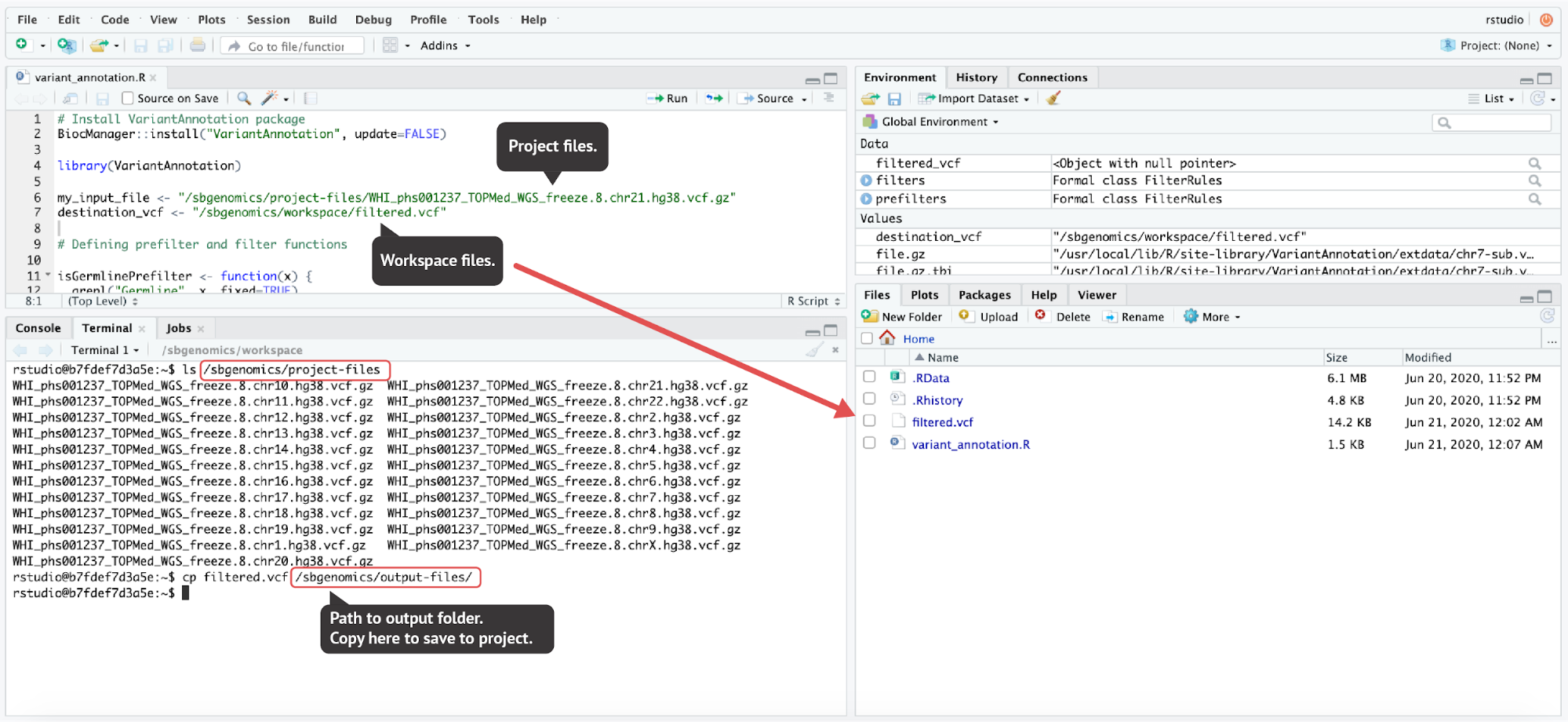
Figure 17. To demonstrate the file handling in RStudio, a simple VCF filtering provided in the VariantAnnotation package vignette was performed. The resulting filtered VCF file was then saved to the project by copying the filtered.vcf file to the output directory (the last command executed in the terminal). The code used here can be found in the Appendix.

Further Reading
Storage Pricing
Users are not charged for files that are part of the hosted datasets on Seven Bridges Platform. Therefore, when users copy the files from the Data Browser, Public Files, or Public Projects to their project, they will not be charged for storing those files.
The files from these hosted datasets are located in cloud buckets maintained by dataset custodians (for the datasets available through the Data Browser) or Seven Bridges (for the files available through Public Files or Public Projects). These files are referenced in the project, while the physical file remains in the corresponding cloud bucket.
A similar logic applies to all files: if a file is copied from one project to another, that file is only referenced in the second project, without creating a physical copy and without additional storage expenses.
If a user creates multiple copies of a file and then deletes the original file, the copies will still remain and the file will still accrue storage costs until ALL copies are deleted.
In addition to computation, storage costs are made up of the cost for storing derived files or any files that a user has uploaded. It is important to note that this applies as long as users keep the files in their projects. To minimize storage costs, it is recommended that users check on their files after each project phase is completed and discard any files which are not needed for the future analysis. Note that intermediate files can always be recreated by running the analysis again, and that the cost to re-create these files can sometimes be less than storing the files in your project.
As noted in the previous paragraph, multiple copies of a file are charged only once. Consequently, when deleting files which have a copy in other projects, users will still accrue storage expenses until all the copies are removed.
Connect cloud storage buckets to the Platform
While Seven Bridges Platform integrates cloud storage services by default, many researchers and organizations have their own cloud buckets where they keep and organize their data.
Connecting those cloud buckets to the Platform may oftentimes be an efficient way to use the private data on the Platform, while still being able to manage the files and storage costs in a centralized way. To establish this connection, we use a feature called Volume.
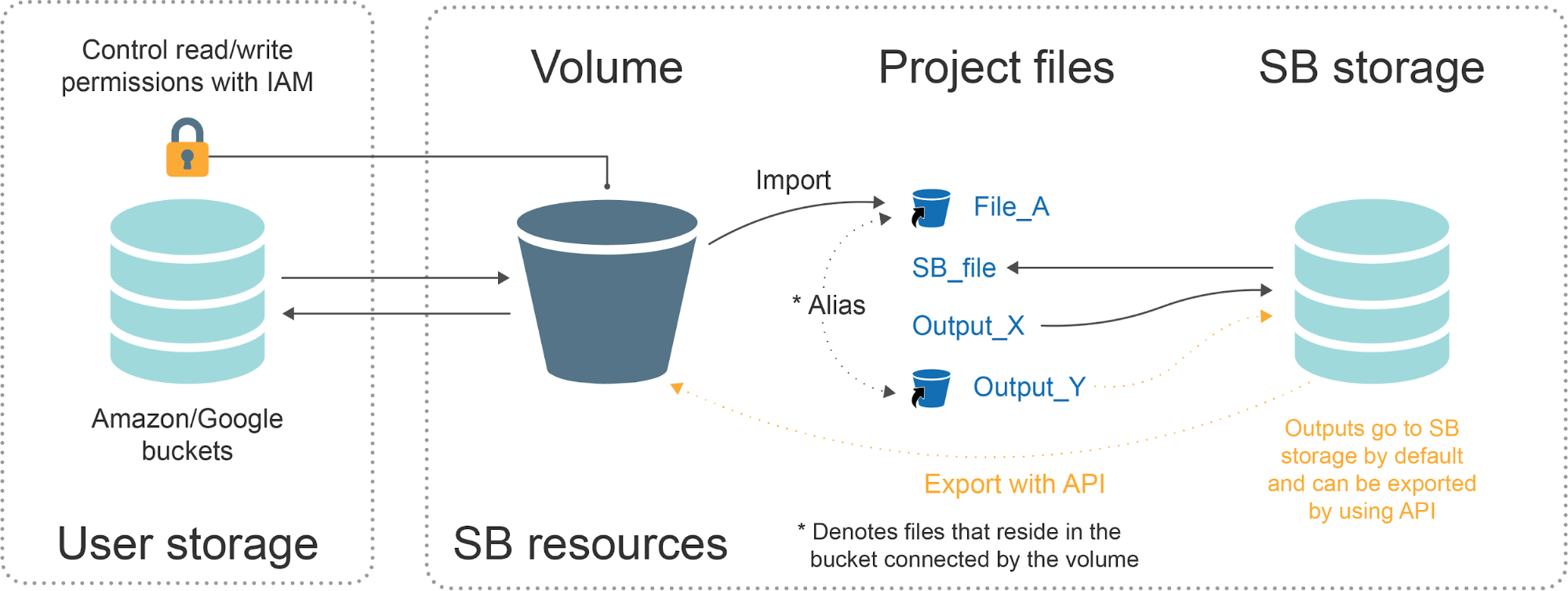
Figure 24: Graphical representation of the connection between the Platform and the storage bucket mediated by the Volume.
Simply put: the Volumes act as an intermediate layer between the bucket content and the actions performed on this content via Platform.
The Seven Bridges Platform supports connection to Google Cloud Storage (GCS) and Amazon Web Services (AWS) S3 buckets and both can be registered as Volumes through an IAM user. The actual steps for establishing a connection are slightly different for different cloud providers and can be accessed here:
Once the connection is successfully created, the Volume that contains all the files found within the bucket will appear on the Platform under Data → Volumes (see Figure 25). From there, you can click on the Volume name to browse the files that you want to import to your project(s).
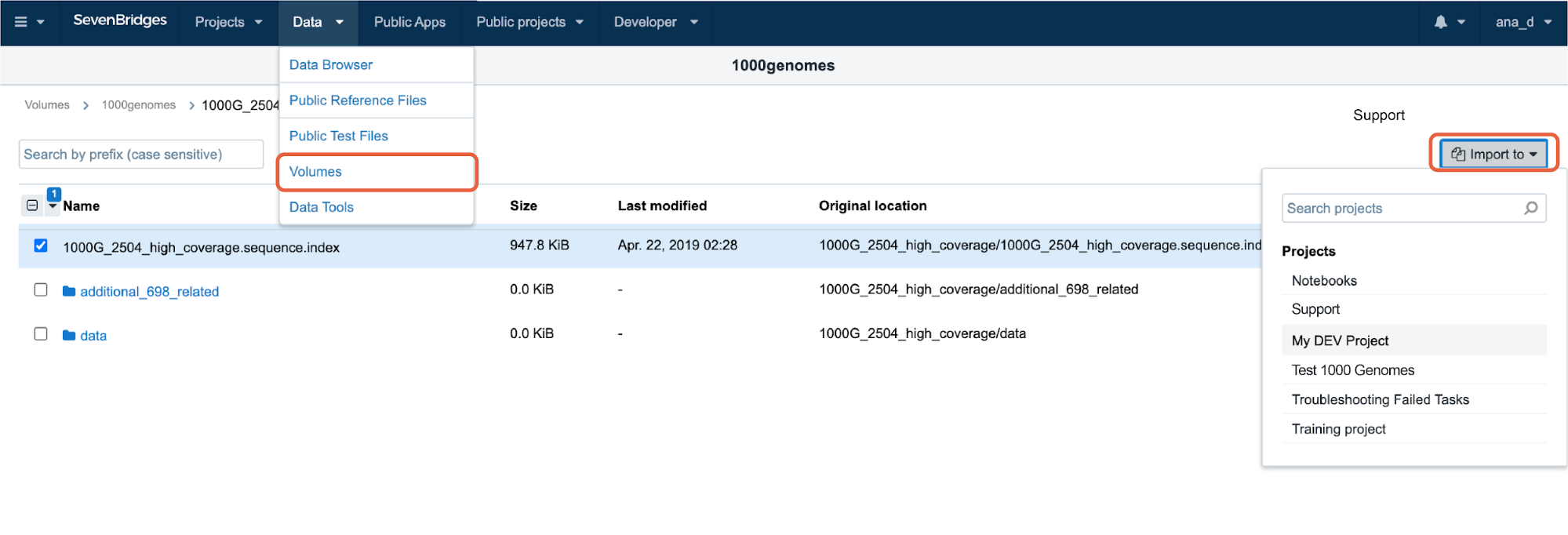
Figure 25: Exploring the bucket content within the Volume and picking files to import into the project.
The files of interest are imported into the projects as aliases (see blue bucket icons in Figure 23). An alias is simply a pointer to a file that resides in the bucket and it enables those external files to be manipulated on the Platform in the same way as any other files that are already present on the Platform, e.g. copying the files across projects, providing them as task inputs, adding the metadata etc.
This also means that the files are not copied to the platform, but only linked. It’s worth reminding that all the rules explained at the beginning of the Storage Pricing section apply here too.
By default, files that are produced on the Platform (e.g. by running tasks) are stored in the Platform storage. Similarly, when those files are exported, they become aliases and get the blue bucket icon next to them (Output_Y file in Figure 23).
Please note that files can be exported to a bucket only via API and through the Seven Bridges CLI (more information is available in above-mentioned instructions).
For questions regarding moving files to manage storage costs, please contact us at [email protected] to learn more about streamlining the process. For more information about storage and computational pricing, visit our Knowledge Center.
Appendix
Using the API
There are numerous use cases in which users might want to have more control over their analysis flow, or simply want to automate custom, repetitive tasks. Even though there are a lot of features enabled through the Platform GUI, certain types of actions will benefit from using the API.
In the section below, a couple of API Python code snippets are provided for the purpose of this manual. These code snippets have proven to be useful for mitigating most frequent obstacles when running large scale analyses on the Seven Bridges Platform.
Before you proceed, note that all examples are written in Python 3.
Install and configure
To be able to run Seven Bridges API, users should first install sevenbridges-python package:
$ pip install sevenbridges-pythonAfter that, users can then experiment with some basic commands to get a sense of how the Seven Bridges API works. To be able to initialize the sevenbridges library, each user will need to generate your authentication token and plug it into the following code:
import sevenbridges as sbg
api = sbg.Api(url='https://api.sbgenomics.com/v2', token='<TOKEN_HERE>')Managing tasks
Here is a simple code for fetching all task IDs from a given project and printing the status metrics:
import collections
# First you need to provide project slug from its URL:
test_project = 'username/project_name'
# Query all the tasks in the project
# Query commands may be time sensitive if there is a huge number of expected items
# See explanation below
tasks = api.tasks.query(project=test_project).all()
statuses = [t.status for t in list(tasks)]
# counter method counts the number of different elements in the list
counter = collections.Counter(statuses)
for key in counter:
print(key, counter[key])The previous code should result in an output similar to this:
ABORTED 1
COMPLETED 8
FAILED 1
DRAFT 1In order to fully understand this section, it is vital to provide an explanation of API rate limits.
The maximum number of API requests, i.e. commands performed on the api object, is 1000 requests in 5 minutes (300 seconds). An example of this command is the following line from the previous code snippet:
tasks = api.tasks.query(project=test_project).all()By default, 50 tasks will be fetched in one request. However, the maximum number is 100 tasks which can be configured as follows:
tasks = api.tasks.query(project=test_project, limit=100).all()In other words, users typically would have to wait for the renewed requests for a couple of minutes if the project contained more than 100,000 tasks. This scenario almost never happens in practice because it is unusual to have this number of tasks in one project. However, this number can be easily reached for the project files to which the same rule applies. The next few code examples illustrate handling files.
Managing files
In this section, methods to perform some simple actions on the project files are described. The following code will print out the number of files in the project:
files = api.files.query(project=test_project, limit=100).all()
len(list(files))In order to now read the metadata of a specific file, the following code can be run:
files = api.files.query(project=test_project, limit=100).all()
for f in list(files):
if f.name == 'CCLE-HCC1143BL-DNA-10_Illumina.converted.pe_1.fastq':
my_meta = f.metadata
for key in my_meta:
print(key,':', my_meta[key])The output appear similar to:
species : Homo sapiens
sample_id : HCC1143BL
case_id : CCLE-HCC1143BL
investigation : CCLE-BRCA
sample_type : EBV Immortalized Normal
experimental_strategy : WGS
paired_end : 1
platform : Illumina
sbg_public_files_category : testAs previously mentioned, the majority of publicly available datasets have immutable metadata. However, if a user has their own files and want to add or modify metadata, they can do so by using the following template:
files = api.files.query(project=test_project, limit=100).all()
for f in list(files):
if f.name == 'example.bam':
f.metadata['foo'] = 'bar'
f.save()
my_meta = f.metadata
for key in my_meta:
print(key,':', my_metadata[key])Bulk operation: reducing the number of requests
By inspection, it is evident that f.save() is called each time it is desired to update the file with new information. This means that the new API request is generated, and recalling that there is a limit of 1,000 requests in 5 minutes, it is evident that this number is easily reached. To solve this issue, bulk methods are used:
all_files = api.files.query(project=test_project, limit=100).all()
all_fastqs = [f for f in all_files if f.name.endswith('.fastq')]
changed_files = []
for f in all_fastqs:
if f is not None:
f.metadata['foo'] = 'bar'
changed_files.append(f)
if changed_files:
changed_files_chunks = [changed_files[i:i + 100] for i in range(0, len(changed_files), 100)]
for cf in changed_files_chunks:
api.files.bulk_update(cf)In this example, it was elected to set metadata only for the FASTQ files found within the project. This was performed by triggering only one API request per 100 files – first splitting the list of files into chunks of at most 100 files, and then running the api.files.bulk_update() method on each chunk.
For more information about other useful bulk operations, see this section in the API Python documentation.
Managing batch tasks
Another vital topic to discuss is batch analysis handling. If there had been batch tasks in the project used for printing out task status information in one of the previous examples, the output would not have included metrics which include children tasks statuses as well. To be able to query children tasks, a couple of additions to the API code is needed.
First, a method to check which tasks are batch tasks:
test_project = 'username/project_name'
tasks = api.tasks.query(project=test_project).all()
for task in tasks:
if task.batch:
print(task.name)Typically, users will want to automatically rerun the failed tasks within batch analysis. The following method described how to filter out failed tasks for the given batch analysis and rerun those tasks with an updated app:
test_project = 'username/project_name'
my_batch_id = 'BATCH_ID'
my_batch_task = api.tasks.get(my_batch_id)
batch_failed = list(api.tasks.query(project=test_project,
parent=my_batch_task.id,
status='FAILED',
limit=100).all())
print('Number of failed tasks: ', len(batch_failed))
for task in batch_failed:
old_task = api.tasks.get(task.id)
api.tasks.create(name='RERUN - ' + old_task.name,
project=old_task.project,
# App example: user-name/demo-project/samtools-depth/8
# You can copy this string from the app's URL
app=api.apps.get('username/project_name/app_name/revision_number'),
inputs=old_task.inputs,
run=True)
print('Running: RERUN - ' + old_task.name)As shown above, there is no need to specify inputs for each task separately – those inputs can be automatically passed from the old tasks. However, this only relates to re-running the tasks in the same project. If a user desires to re-run the tasks in a different project, they will need to write a couple of additional lines to copy the files and apps.
Collecting specific outputs
The following example is a useful reference for users who have had their analysis completed successfully and wish to fetch and examine further specific outputs.
Detailed below is the code for copying all the files belonging to a particular output node from all successful runs to another project. The code can be easily modified to fit a specific purpose, e.g. renaming the files, deleting them if desired to offload the storage, providing those as inputs to another app, etc.
The code may appear complex because it contains error handling and logging, but the iteration through the tasks and collecting the files of interest is fairly simple.
The resulting code will look like this:
import sevenbridges as sbg
import time
from sevenbridges.http.error_handlers import (rate_limit_sleeper, maintenance_sleeper, general_error_sleeper)
import logging
logging.basicConfig(level=logging.INFO)
time_start = time.time()
my_token = '<INSERT_TOKEN>'
# Copy project slug from the project URL
my_project = 'username/project_name'
# Get output ID from the "Outputs" tab within "Ports" section on the app page
my_output_id = 'output_ID'
api = sbg.Api('https://api.sbgenomics.com/v2', token=my_token, advance_access=True,
error_handlers=[rate_limit_sleeper, maintenance_sleeper, general_error_sleeper])
tasks_queried = list(api.tasks.query(project=my_project, limit=100).all())
task_files_list = []
print('Tasks fetched: ', time.time() - time_start)
for task in tasks_queried:
if task.batch:
ts = time.time()
children = list(api.tasks.query(project=my_project,
parent=task.id,
status='COMPLETED',
limit=100).all())
print('Query children tasks', time.time() - ts)
for t in children:
task_files_list.append(t.outputs[my_output_id])
elif task.status == 'COMPLETED':
task_files_list.append(task.outputs[my_output_id])
print('Outputs from all tasks collected: ', time.time() - time_start, '\n')
fts = time.time()
for f in task_files_list:
print('Copying: ', f.name)
# Uncomment the following line if everything works as expected after inserting your values
# f.copy(project='username/destination_project')
print('\nAll files copied :', time.time() - fts)
print('All finished: ', time.time() - time_start)There are two major cases: (1) either users run into the batch task in which case it is required to iterate through all completed children tasks, or (2) users simply run into an individual task and only need to check if the status is “COMPLETED”.
If any of these two conditions are satisfied, then the output of interest is collected (lines 17 to 28) and then loop through those to copy the files (lines 31 to 34).
For any questions or if help with using API is needed, please contact support.
RStudio VariantAnnotation Example
For users who would like to test the RStudio environment in Data Studio, the following code can be quickly run. Alternatively, users can adjust the code to take some other VCF as input.
In that case, creating a VCF tabix index in advance (by using Tabix Index app) and plugging its path into the code below is required.
# Install VariantAnnotation package
BiocManager::install("VariantAnnotation", update=FALSE)
library(VariantAnnotation)
destination_vcf <- "/sbgenomics/workspace/filtered.vcf"
# Defining prefilter and filter functions
isGermlinePrefilter <- function(x) {
grepl("Germline", x, fixed=TRUE)
}
notInDbsnpPrefilter <- function(x) {
!(grepl("dbsnp", x, fixed=TRUE))
}
## We will use isSNV() to filter only SNVs
allelicDepth <- function(x) {
## ratio of AD of the "alternate allele" for the tumor sample
## OR "reference allele" for normal samples to total reads for
## the sample should be greater than some threshold (say 0.1,
## that is: at least 10% of the sample should have the allele
## of interest)
ad <- geno(x)$AD
tumorPct <- ad[,1,2,drop=FALSE] / rowSums(ad[,1,,drop=FALSE])
normPct <- ad[,2,1, drop=FALSE] / rowSums(ad[,2,,drop=FALSE])
test <- (tumorPct > 0.1) | (normPct > 0.1)
as.vector(!is.na(test) & test)
}
prefilters <- FilterRules(list(germline=isGermlinePrefilter, dbsnp=notInDbsnpPrefilter))
filters <- FilterRules(list(isSNV=isSNV, AD=allelicDepth))
file.gz <- system.file("extdata", "chr7-sub.vcf.gz", package="VariantAnnotation")
file.gz.tbi <- system.file("extdata", "chr7-sub.vcf.gz.tbi", package="VariantAnnotation")
# Uncomment the following two lines and insert paths to your files
# file.gz <- "/sbgenomics/project-files/WHI_phs001237_TOPMed_WGS_freeze.8.chr21.hg38.vcf.gz"
# file.gz.tbi <- "/sbgenomics/project-files/WHI_phs001237_TOPMed_WGS_freeze.8.chr21.hg38.vcf.gz.tbi"
tabix.file <- TabixFile(file.gz, yieldSize=10000)
filterVcf(tabix.file, "hg19", destination_vcf, prefilters=prefilters, filters=filters, verbose=TRUE)
filtered_vcf <- readVcf(destination_vcf, "hg19")For more information on the VariantAnnotation package, visit the Bioconductor page here.
Updated 3 months ago